photos
Check out photos of what’s possible with RINVOQ, a once-daily pill for moderate to severe eczema (atopic dermatitis).
Learn how AbbVie could help you save. 1-800-2RINVOQ
For adults and children 12+ years with moderate to severe eczema (atopic dermatitis) that did not respond to previous treatment and their eczema is not well controlled using other pills or injections, including biologics, or the use of other pills or injections is not recommended.
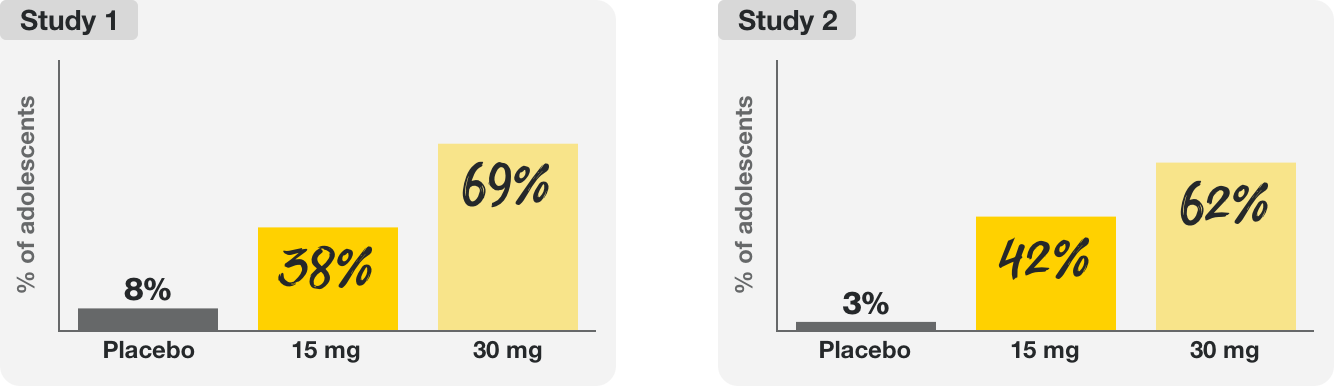
Two 16-week clinical trials tested the efficacy and safety of RINVOQ 15 mg and RINVOQ 30 mg in over 200 adolescents (ages 12–17) with moderate to severe eczema (atopic dermatitis).
In these trials, RINVOQ helped provide:

Many adolescents taking RINVOQ had significant improvement in itch at 16 weeks.

The majority of adolescents taking RINVOQ saw 75% clearer skin, and many saw clear or almost-clear skin at 16 weeks.
% of adolescents who achieved
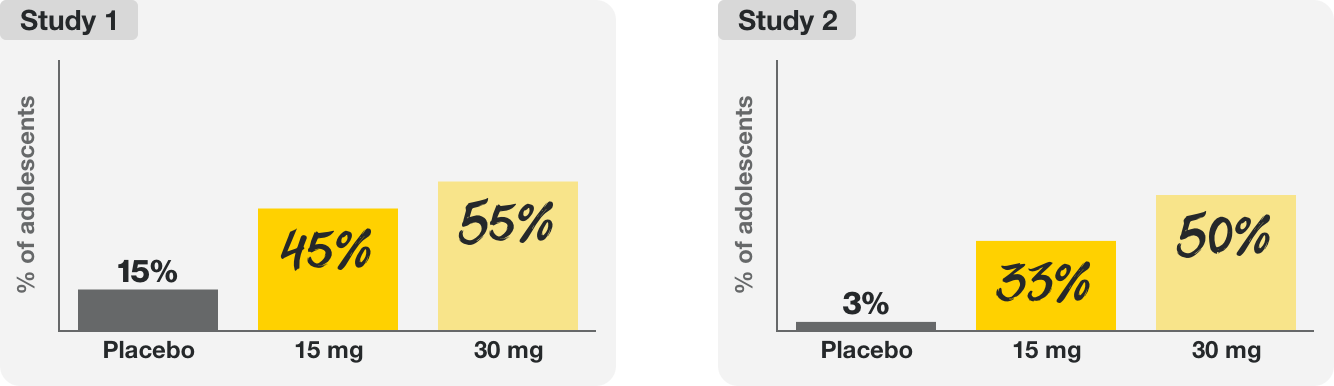
% of adolescents who achieved
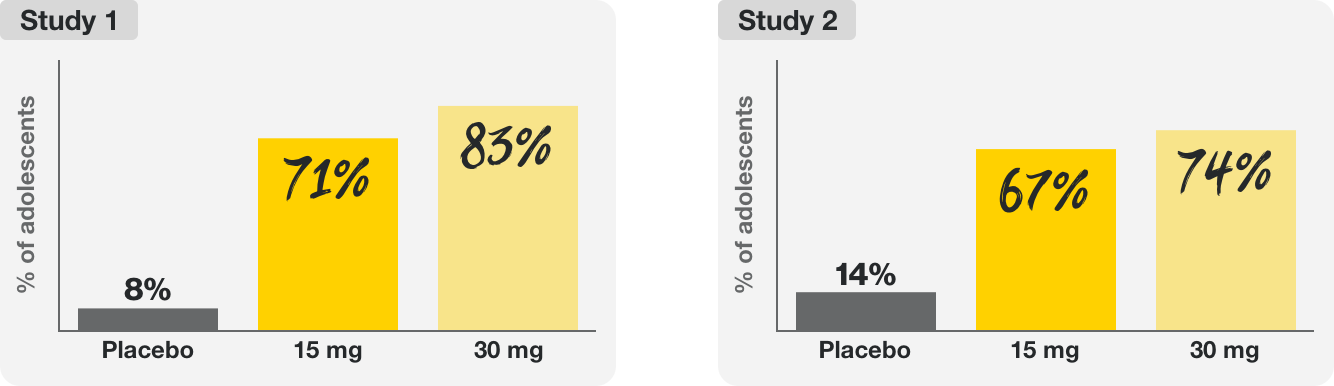
% of adolescents who achieved
The approved starting dose for RINVOQ is 15 mg. In certain cases, your dermatologist may consider increasing to 30 mg. Your dermatologist will prescribe the dose that's right for you. Take RINVOQ exactly as prescribed.
Select Important Safety Information
RINVOQ may cause serious side effects, including:
Safety Considerations
RINVOQ may cause serious side effects, including:
Learn more about these and the full Important Safety Information below.

RINVOQ helps disrupt the unrelenting itch and rash of moderate to severe eczema from inside the body—here’s how.

Dig into

photos
Check out photos of what’s possible with RINVOQ, a once-daily pill for moderate to severe eczema (atopic dermatitis).
RINVOQ is a prescription medicine used to treat adults and children 12 years of age and older with moderate to severe eczema (atopic dermatitis) that did not respond to previous treatment and their eczema is not well controlled with other pills or injections, including biologic medicines, or the use of other pills or injections is not recommended. It is not known if RINVOQ is safe and effective in children under 12 years of age with atopic dermatitis.
IMPORTANT SAFETY INFORMATION FOR RINVOQ/RINVOQ LQ (upadacitinib)
What is the most important information I should know about RINVOQ**?
RINVOQ may cause serious side effects, including:
Do not take RINVOQ if you are allergic to upadacitinib or any of the ingredients in RINVOQ. See the Medication Guide or Consumer Brief Summary for a complete list of ingredients.
What should I tell my HCP BEFORE starting RINVOQ?
Tell your HCP if you:
Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. RINVOQ and other medicines may affect each other, causing side effects.
Especially tell your HCP if you take:
If you are not sure if you are taking any of these medicines, ask your HCP or pharmacist.
What should I avoid while taking RINVOQ?
Avoid food or drink containing grapefruit during treatment with RINVOQ as it may increase the risk of side effects.
What should I do or tell my HCP AFTER starting RINVOQ?
What are other possible side effects of RINVOQ?
Common side effects include upper respiratory tract infections (common cold, sinus infections), shingles (herpes zoster), herpes simplex virus infections (including cold sores), bronchitis, nausea, cough, fever, acne, headache, swelling of the feet and hands (peripheral edema), increased blood levels of creatine phosphokinase, allergic reactions, inflammation of hair follicles, stomach-area (abdominal) pain, increased weight, flu, tiredness, lower number of certain types of white blood cells (neutropenia, lymphopenia, leukopenia), muscle pain, flu-like illness, rash, increased blood cholesterol levels, increased liver enzyme levels, pneumonia, low number of red blood cells (anemia), and infection of the stomach and intestine (gastroenteritis).
A separation or tear to the lining of the back part of the eye (retinal detachment) has happened in people with atopic dermatitis treated with RINVOQ. Call your HCP right away if you have any sudden changes in your vision during treatment with RINVOQ.
Some people taking RINVOQ may see medicine residue (a whole tablet or tablet pieces) in their stool. If this happens, call your HCP.
These are not all the possible side effects of RINVOQ.
How should I take RINVOQ/RINVOQ LQ?
RINVOQ is taken once a day with or without food. Do not split, crush, or chew the tablet. Take RINVOQ exactly as your HCP tells you to use it. RINVOQ is available in 15 mg, 30 mg, and 45 mg extended-release tablets. RINVOQ LQ is taken twice a day with or without food. RINVOQ LQ is available in a 1 mg/mL oral solution. RINVOQ LQ is not the same as RINVOQ tablets. Do not switch between RINVOQ LQ and RINVOQ tablets unless the change has been made by your HCP.
**Unless otherwise stated, "RINVOQ" in the IMPORTANT SAFETY INFORMATION refers to RINVOQ and RINVOQ LQ.
This is the most important information to know about RINVOQ. For more information, talk to your HCP.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
USES
RINVOQ is a prescription medicine used to treat:
It is not known if RINVOQ is safe and effective in children with ankylosing spondylitis, non-radiographic axial spondyloarthritis, ulcerative colitis, or Crohn's disease.
It is not known if RINVOQ is safe and effective in children under 12 years of age with atopic dermatitis.
It is not known if RINVOQ LQ is safe and effective in children with atopic dermatitis.
RINVOQ/RINVOQ LQ is a prescription medicine used to treat:
It is not known if RINVOQ/RINVOQ LQ is safe and effective in children under 2 years of age with polyarticular juvenile idiopathic arthritis or psoriatic arthritis.
If you are having difficulty paying for your medicine, AbbVie may be able to help. Visit AbbVie.com/PatientAccessSupport to learn more.
US-RNQ-250009
Please see the Full Prescribing Information, including the Medication Guide, for RINVOQ.
Legal Notices. © 2025 AbbVie. All rights reserved. If you have any questions about AbbVie's RINVOQ.com website that have not been answered, click here. This website and the information contained herein is intended for use by US residents only, is provided for informational purposes only and is not intended to replace a discussion with a healthcare provider. All decisions regarding patient care must be made with a healthcare provider and consider the unique characteristics of each patient.