with RINVOQ
For adults with moderate to severe Crohn's disease in whom a TNF blocker did not work well, or after a different injection or pill when TNF blockers are not recommended by your healthcare provider
RINVOQ results that won’t back down

In clinical studies, RINVOQ helped people achieve remission at 12 weeks and 1 year.
Plus, RINVOQ helped deliver:

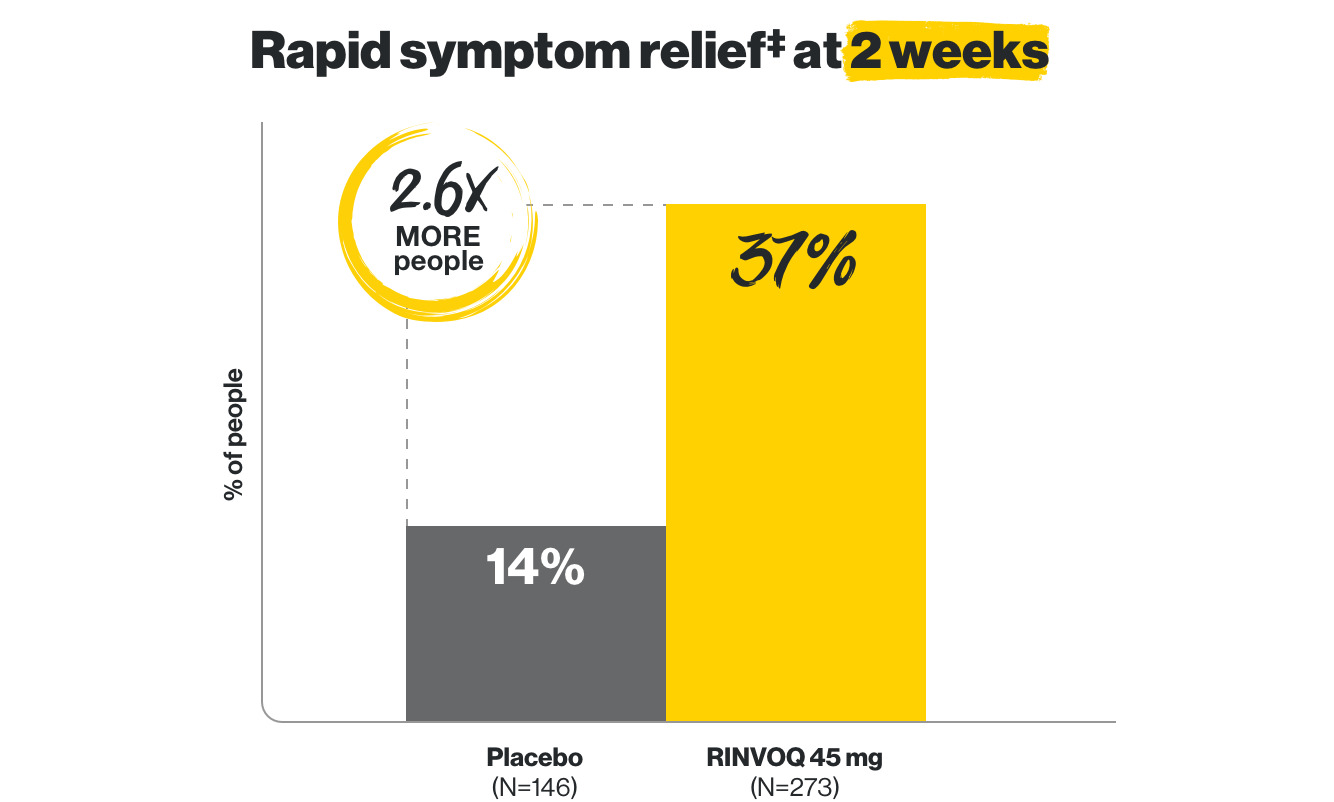
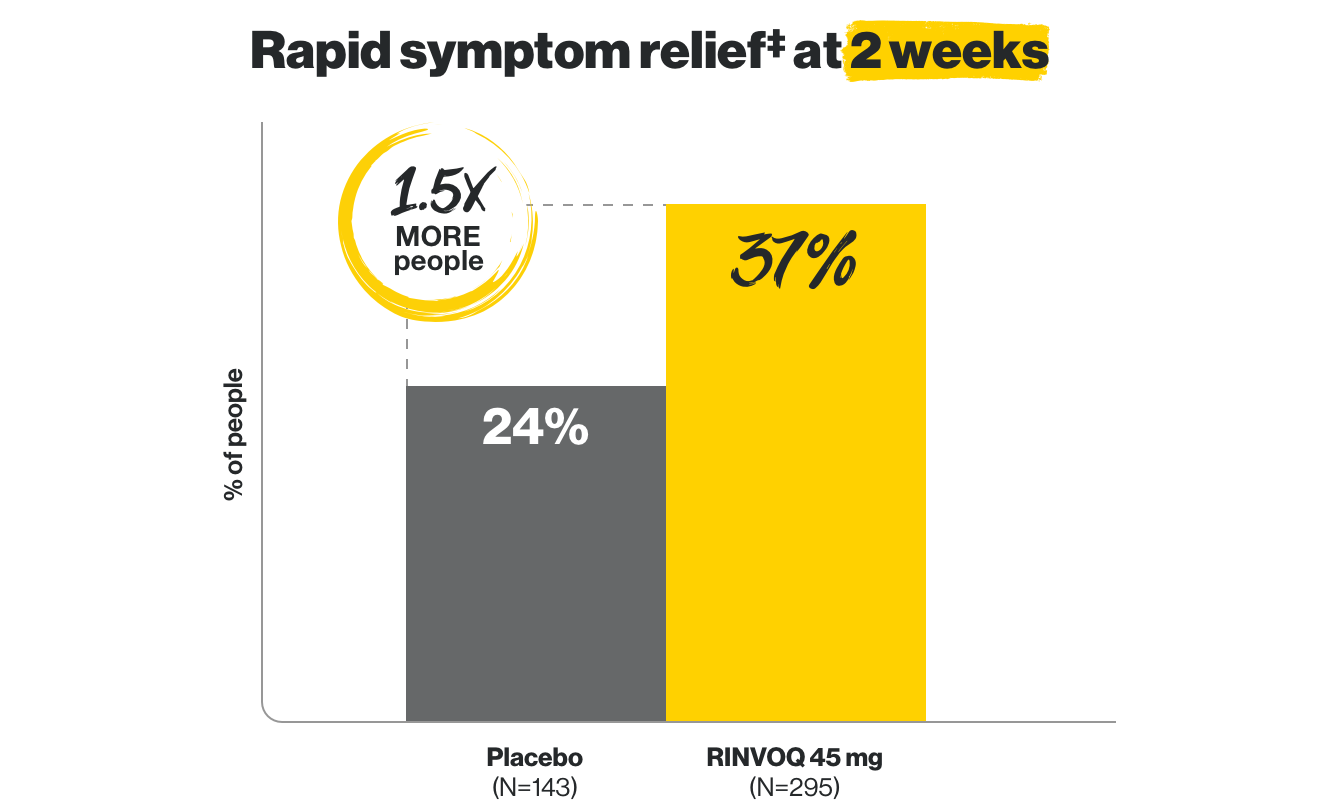
Rapid symptom relief
including less abdominal pain and fewer bowel movements in as early as 2 weeks

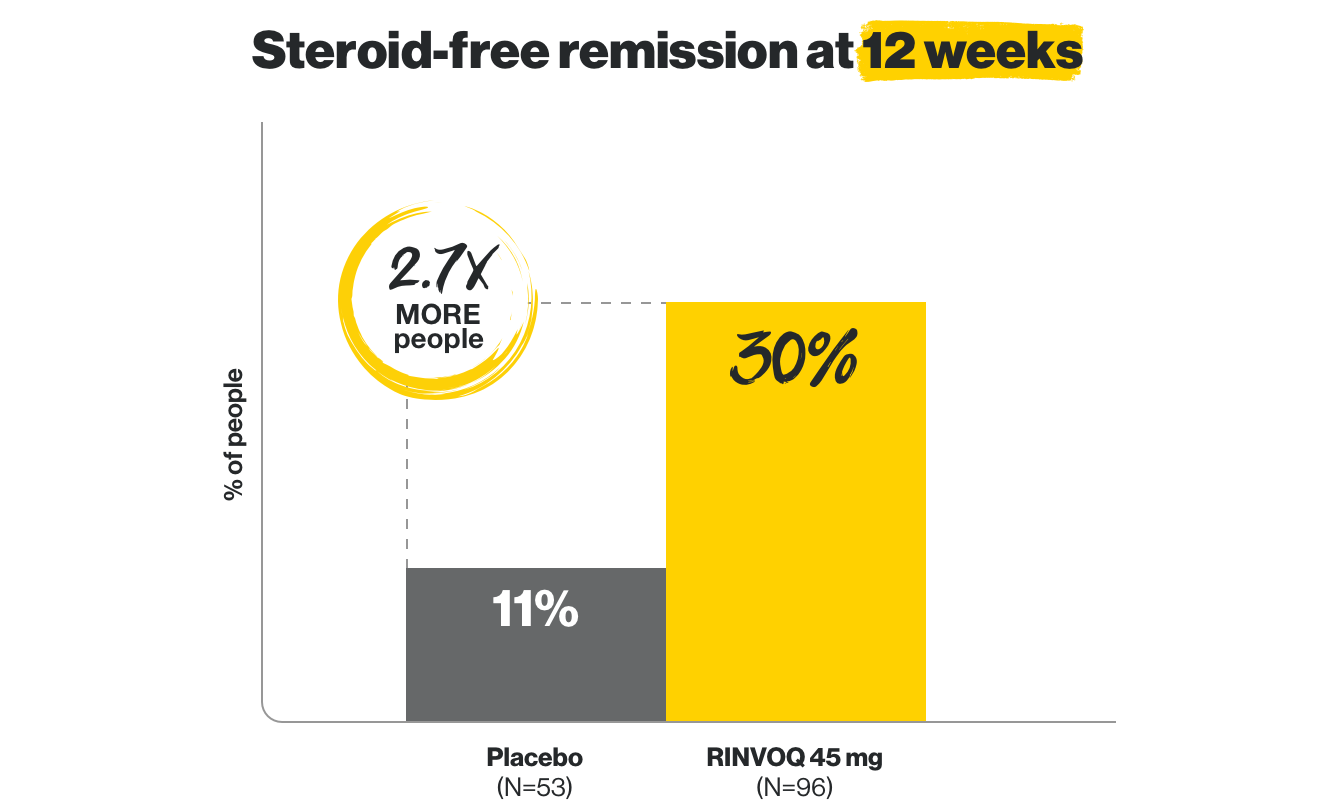
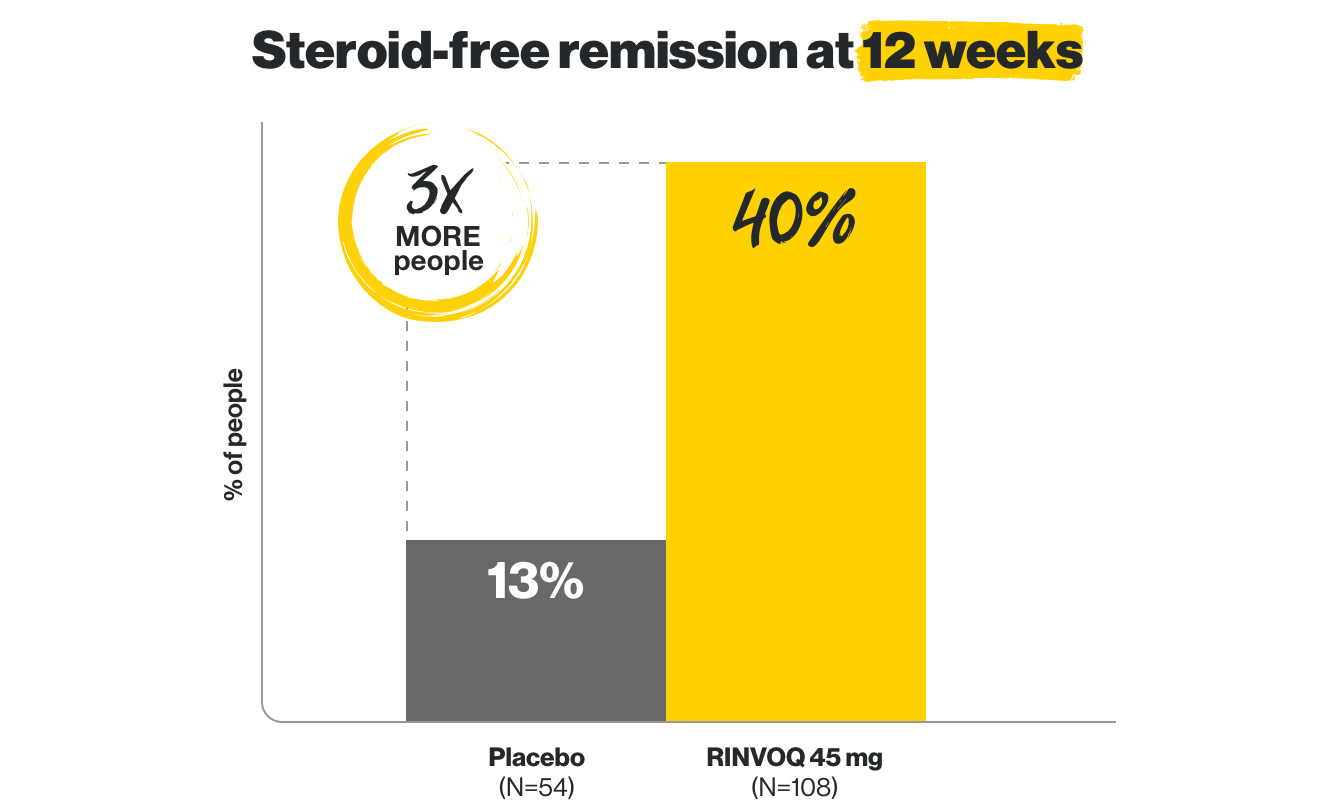
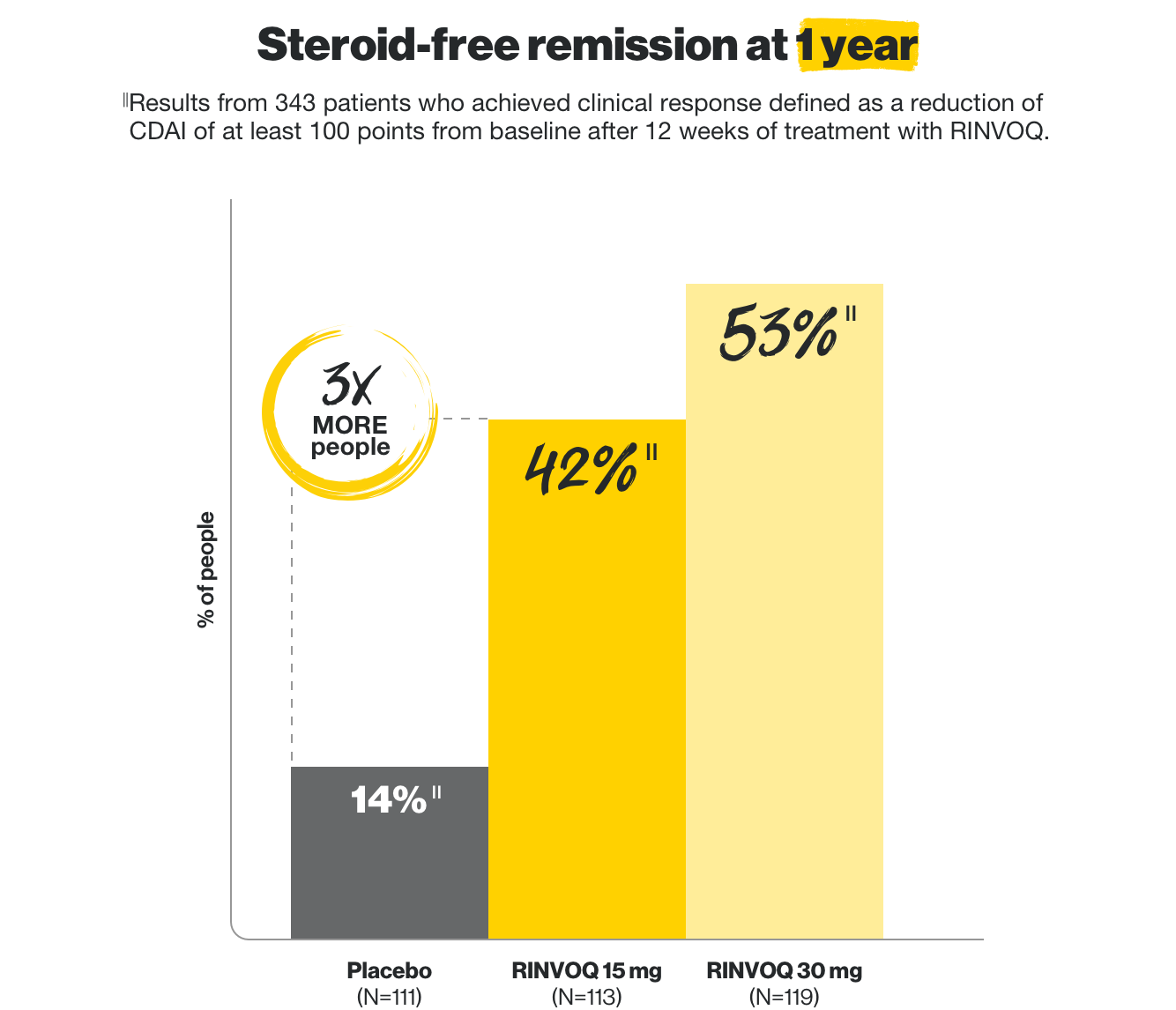
Early remission without steroids
at Week 12, and lasting, steroid-free remission at 1 year

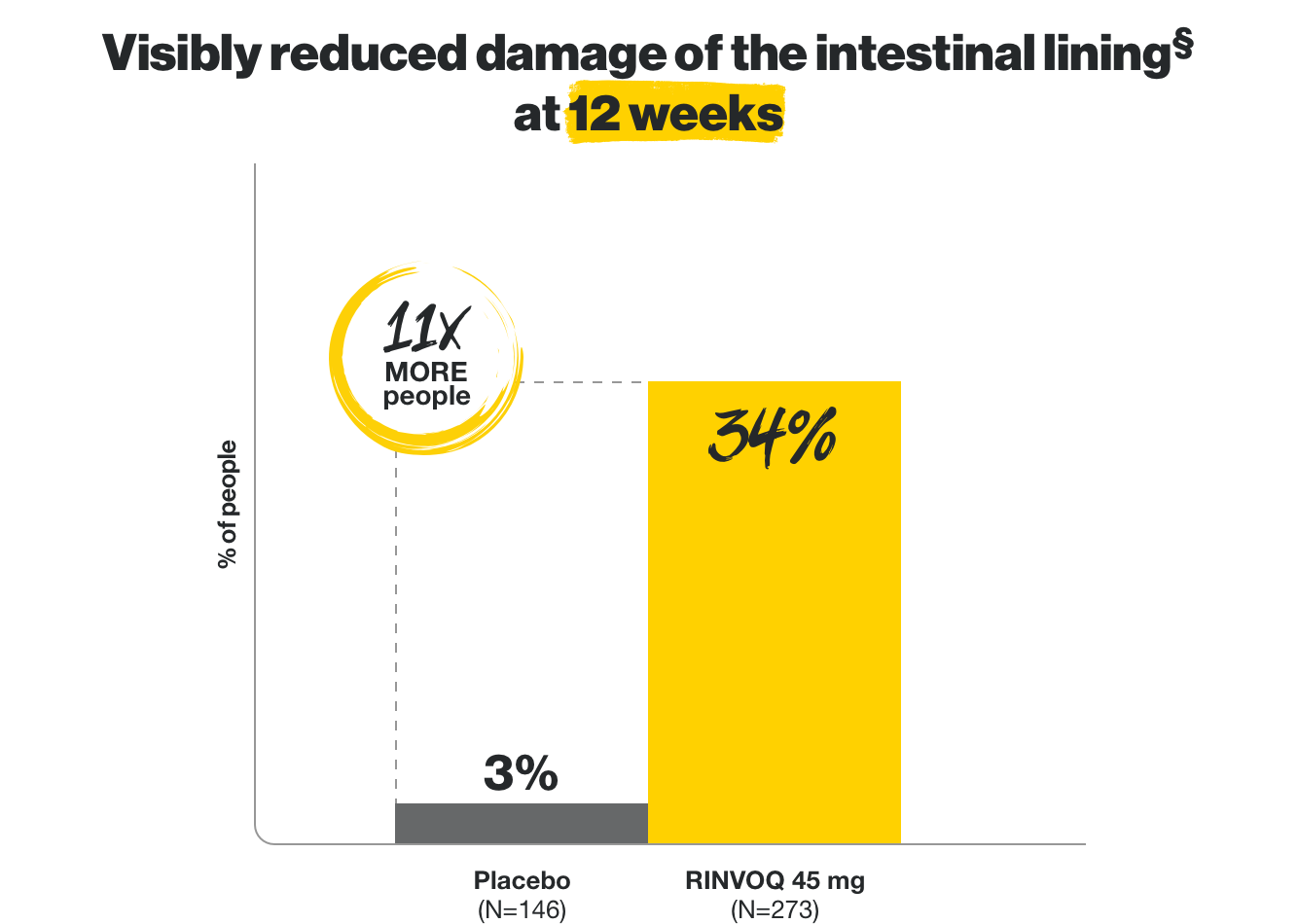
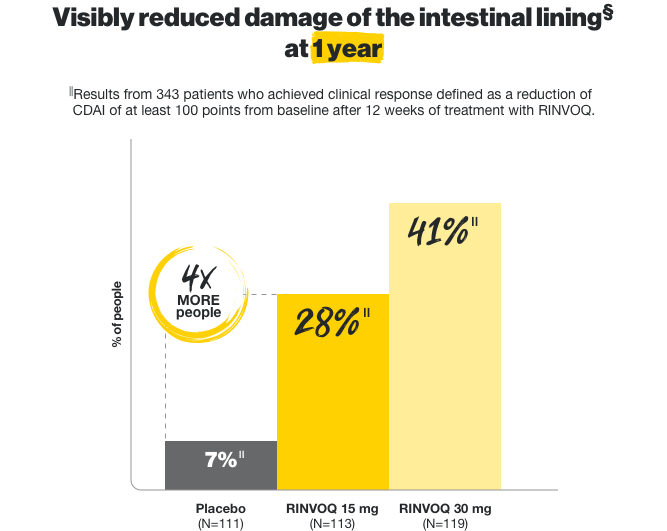
Visibly reduced damage
of the intestinal lining*
*Based on endoscopy at 12 weeks and 1 year.

Significant reduced fatigue
at 12 weeks

Long-term results
In another study, many people were in remission, even at ~3 years†
†In a less rigorous study with data available at ~3 years, physicians and patients knew that RINVOQ was being used, which may have influenced results. Results measured in RINVOQ-treated patients who met clinical response (decrease of CDAI score by ≥100 points) at 12 weeks.

Rapid symptom relief
including less abdominal pain and fewer bowel movements in as early as 2 weeks

Early remission without steroids
at Week 12, and lasting steroid-free remission at 1 year

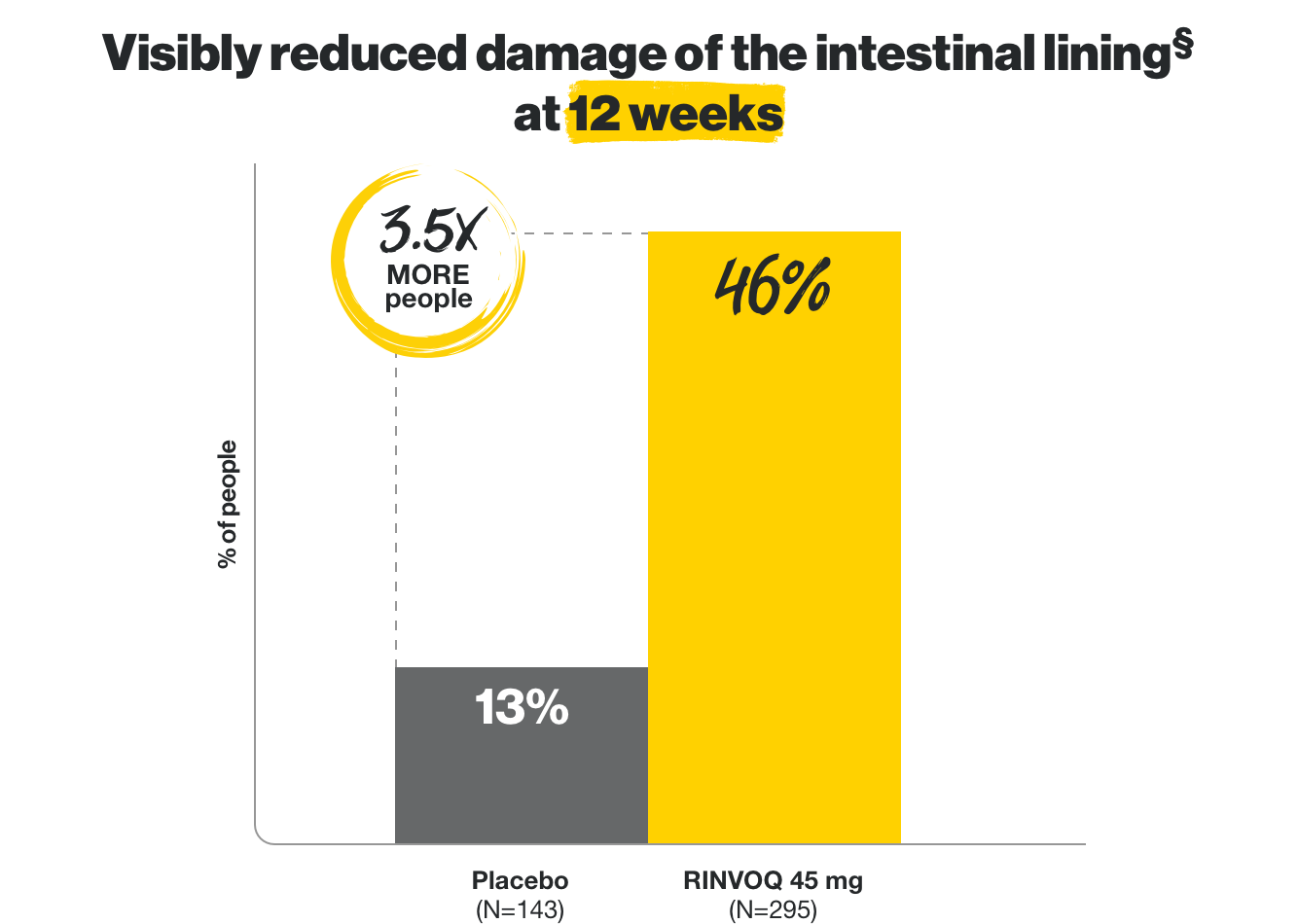
Visibly reduced damage
of the intestinal lining*
*Based on endoscopy at 12 weeks and 1 year.

Significantly reduced fatigue
at 12 weeks

Long-term results
In another study, many people were in remission, even at ~3 years†
†In a less rigorous study with data available at ~3 years, physicians and patients knew that RINVOQ was being used, which may have influenced results. Results measured in RINVOQ-treated patients who met clinical response (decrease of CDAI score by ≥100 points) at 12 weeks.
In two clinical trials, some patients who achieved clinical response on RINVOQ at 12 weeks entered into a maintenance trial. Of those who entered, RINVOQ helped these patients achieve these results at 1 year.
Click on the tabs below to see RINVOQ results

SAFETY CONSIDERATIONS
RINVOQ may cause serious side effects, including:
- Serious infections. RINVOQ can lower ability to fight infections. Serious infections, some fatal, occurred, including tuberculosis (TB) and infections caused by bacteria, fungi, or viruses.
- Increased risk of death in people age 50+ with at least 1 heart disease risk factor.
- Cancer and immune system problems. Increased risk of some cancers, including lymphoma and skin. Current or past smokers have higher risk for lymphoma and lung cancer.
- Increased risk of major cardiovascular events such as heart attack, stroke, or death in people 50+ with at least 1 heart disease risk factor, especially in current or past smokers.
- Blood clots, some fatal, in veins of the legs or lungs and arteries. This occurred more often in people 50+ with at least 1 heart disease risk factor.
- Serious allergic reactions. Do not take if allergic to RINVOQ or its ingredients.
- Tears in the stomach or intestines; changes in certain laboratory test results.
Learn more about these and the full Important Safety Information below.
Keep scrolling for endoscopy images from real patients in RINVOQ clinical trials at 12 weeks and 1 year

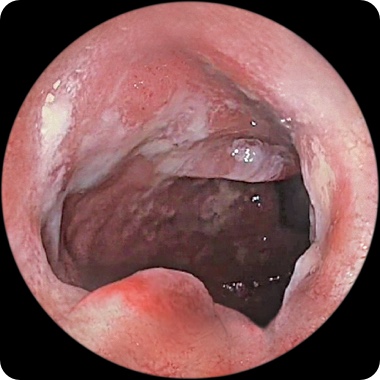
PATIENT 1
Endoscopy images from a real clinical trial patient. Adult patient with moderate to severe Crohn's disease received RINVOQ 45 mg (induction) and 15 mg (maintenance), once daily. Patient had previously used a medicine called a tumor necrosis factor (TNF) blocker that either did not work well or could not be tolerated.
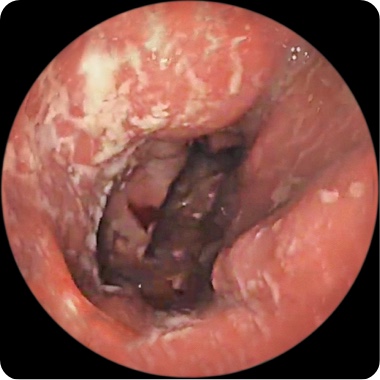
Before RINVOQ
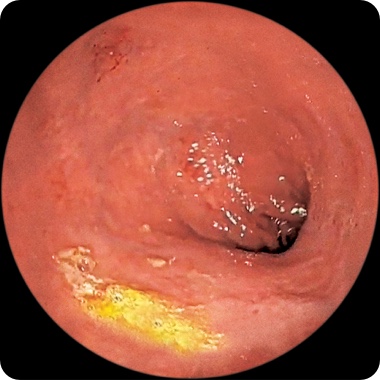
Week 12
1 Year
This patient achieved endoscopic remission at Week 12 and also at 1 year. In clinical trials, some patients on RINVOQ experienced: endoscopic remission§ (little to no visible evidence of active Crohn's) at 12 weeks and 1 year. Results at 1 year were measured in RINVOQ-treated patients who met clinical response (decrease of CDAI score by ≥100 points) at 12 weeks.
Individual results may vary.
§Based on areas that were assessed with endoscopy.
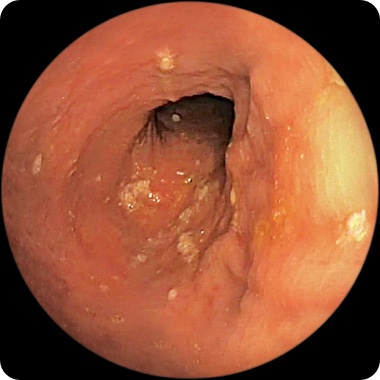
PATIENT 2
Endoscopy images from a real clinical trial patient. Adult patient with moderate to severe Crohn's disease received RINVOQ 45 mg (induction) and 30 mg (maintenance), once daily. Patient had previously used a medicine called a tumor necrosis factor (TNF) blocker that either did not work well or could not be tolerated.
Before RINVOQ
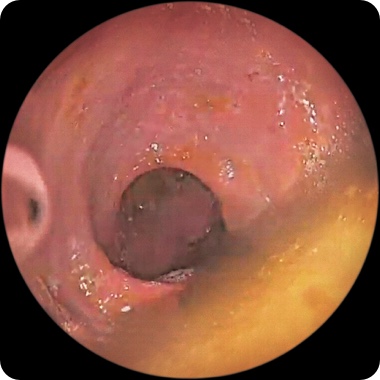
Week 12
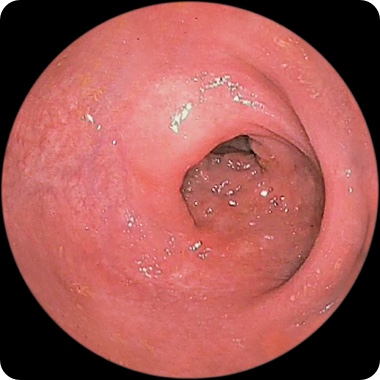
1 Year
This patient achieved endoscopic response at Week 12 and also at 1 year. See RINVOQ clinical trial results for visibly reduced damage of the intestinal lining§ above.
Individual results may vary.
Your doctor will decide which maintenance dose (15 mg or 30 mg) is right for you.
§Based on areas that were assessed with endoscopy.

Understanding the possible side effects of RINVOQ
Consider the benefits and risks of taking RINVOQ to make an informed treatment choice with your gastroenterologist.

Now that my symptoms are under control, I’m able to plan more things with my daughter and just be present and in the moment with her.
—Julie, moderate to severe Crohn’s disease patient

REMICADE® (infliximab) is a registered trademark of Janssen Biotech, Inc.

Is your current treatment meeting your goals?
Use our discussion tool to help start a conversation with your doctor.

Looking for a specialist to help treat your Crohn’s disease?
Find a gastroenterologist near you.