WITH ACTIVE POLYARTICULAR JUVENILE IDIOPATHIC ARTHRITIS



WITH ACTIVE
POLYARTICULAR JUVENILE
IDIOPATHIC ARTHRITIS
Not an actual patient.
For children 2+ years with active polyarticular juvenile idiopathic arthritis in whom TNF blockers did not work well.

About polyarticular
juvenile idiopathic arthritis (pJIA)
Polyarticular juvenile idiopathic arthritis is a chronic (ongoing) condition. That means symptoms may keep coming back and last a long time. Once someone has pJIA, they may always have it. Caring for a child with a chronic condition presents unique challenges. Learn more with these caregiver resources.
Front of body
What is inflammation?
The causes of pJIA are unknown. Some children with pJIA have an overactive immune system, which can lead to inflammation. This inflammation causes symptoms such as joint pain, swelling, and stiffness.
Inflammation is the body’s normal response to protect against something the immune system thinks will cause harm. When excess inflammation caused by the immune system doesn’t go away, it can lead to conditions such as pJIA and symptoms including:
- Painful, swollen, and tender joints
- Joint stiffness that worsens in the morning
Helpful tips for managing pJIA
Here are some steps you can take to help manage your child’s pJIA symptoms.
Taking
RINVOQ/RINVOQ LQ
It’s important to remember that pJIA is a chronic, or ongoing, condition. Giving RINVOQ/RINVOQ LQ to your child as prescribed is important because pJIA can persist even if they are not experiencing symptoms.
RINVOQ is available as RINVOQ LQ oral solution (a twice-daily liquid), or RINVOQ tablets (a once-daily pill). The exact dosing is based on your child's weight. RINVOQ/RINVOQ LQ must be given exactly as prescribed by their doctor. Refer to the Instructions for USE on how to prepare and give a dose of RINVOQ LQ oral solution. RINVOQ LQ oral solution is not interchangeable or substitutable with RINVOQ tablets.
RINVOQ LQ twice daily oral solution
Children weighing at least 22 pounds (10 kg) may be prescribed RINVOQ LQ oral solution.
- Give RINVOQ LQ oral solution twice daily, with or without food. Avoid food or drink containing grapefruit
- Do not dilute or mix the oral solution
RINVOQ once-daily pill
Children weighing greater than 66 pounds (30 kg) may be prescribed either RINVOQ LQ oral solution or RINVOQ tablets.
- Give a RINVOQ pill once daily, with or without food. Avoid food or drink containing grapefruit
- Do not split, crush, or chew the pill
Call your child's healthcare provider if you see medication residue (a whole tablet or tablet pieces) in their stool.
What should you do if your child misses a dose?
- Call your child’s doctor about the missed dose
- Set up a visual cue or reminder that will help you and/or your child remember to take RINVOQ/RINVOQ LQ
- Use treatment reminders and fill out your child’s treatment log in the RINVOQ Complete App, so you’ll know when/if your child has taken RINVOQ/RINVOQ LQ
Check in with your child’s doctor
Regular check-ins and open discussions with your child’s doctor about their condition can help them stay on track with their prescribed treatment plan.

Track symptoms
Use the RINVOQ Complete App to keep track of symptoms and share them with your child’s doctor.

Not an actual patient.
How RINVOQ/RINVOQ LQ works
With pJIA, an overactive immune system can lead to inflammation and symptoms including painful, swollen, and tender joints. RINVOQ/RINVOQ LQ works inside cells to block certain signals that are thought to cause inflammation.
Select Important Safety Information for RINVOQ/RINVOQ LQ (upadacitinib)
RINVOQ* may cause serious side effects, including:
- Serious Infections, Cancer and Immune System Problems, and Blood Clots.
- Increased risk of death in people age 50+ with at least 1 heart disease risk factor.
- Increased risk of major cardiovascular events, such as heart attack, stroke, or death in people age 50+ with at least 1 heart disease risk factor, especially in current or past smokers.
- Serious allergic reactions. Do not take if allergic to RINVOQ or its ingredients.
*Unless otherwise stated, “RINVOQ” in the SELECT IMPORTANT SAFETY INFORMATION refers to RINVOQ and RINVOQ LQ.
See full Important Safety Information below.
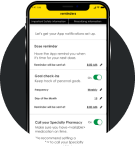
Set reminders
Use the App to set medication reminders and track doses.
Understanding the possible side effects of RINVOQ/RINVOQ LQ
Always consider the benefits and risks of taking RINVOQ/RINVOQ LQ and discuss with your doctor.
Get 1-to-1 support from your RINVOQ Complete Nurse Ambassador*
Reach out when you need answers. Just call 1.800.2RINVOQ (1.800.274.6867).
*Nurse Ambassadors are provided by AbbVie and do not work under the direction of your health care professional (HCP) or give medical advice. They are trained to direct patients to their HCP for treatment-related advice, including further referrals.
Build a routine with the
RINVOQ
Complete App
- Use medication reminders and track doses so you know when/if your child has taken their medicine
- Log and share symptoms with your child’s doctor
- Set personal goals to help you and your child manage their condition, to help them stay motivated
Download the App now
Search for “RINVOQ Complete” at the App Store® or Google Play™.
Text APP to 33101 to receive a link to download
the RINVOQ Complete App
Message and data rates may apply. Visit SeeRTerms.com and see Terms of Use at abbv.ie/s-use. Text STOP to 33101 to unsubscribe. The RINVOQ Complete App can only be registered to a single device.
